The Nobel Prize in Physiology or Medicine 2012 was awarded jointly to Sir John B. Gurdon and Shinya Yamanaka for the discovery that mature cells can be reprogrammed to become pluripotent, i.e. immature cells capable of developing into all tissues of the body. This finding is of revolutionary importance for the field of medicine.

On October 8th 2012, the Nobel Assembly at the Karolinska Institutet in Stockholm announced the names of the winners of the Nobel Prize in Physiology or Medicine 2012. The prize was awarded jointly to the British cell biologist Professor Dr. John B. Gurdon from Cambridge, UK, and the Japanese stem cell researcher Professor Dr. Shinya Yamanaka from the University of Kyoto for the “discovery that mature cells can be reprogrammed to become pluripotent”. The two researchers discovered that mature specialised cells can be reprogrammed to become immature cells capable of developing into any cell type in the body. Their findings have revolutionised our understanding of how cells and organisms develop, was the reason given by the Nobel Assembly for awarding the Nobel Prize to the two researchers. The findings could also revolutionise the field of medicine.
Two generations of researchers

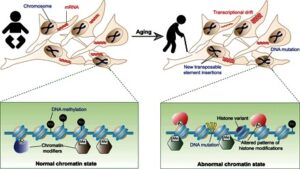
It was previously assumed that the development of immature cells of the early embryo into specialised cells with different functions – nerve, muscle, liver cells, etc. – was a one-way street, i.e. that their differentiation is irreversible. However, we now know that mature cells do not have to be confined forever to their specialised state and that they can be turned back into pluripotent stem cells under certain circumstances. By reprogramming human cells, scientists have created new opportunities to study diseases and develop methods for diagnosis and therapy.
Gurdon and Yamanaka come from two different generations and scientific traditions. 79-year-old Gurdon carried out Xenopus laevis (clawed frog) experiments in 1962 and proved that the genome of a specialised cell still contains all the information needed to enable it to develop into all the different cell types of an organism. Using a micropipette, Gurdon removed cell nuclei derived from mature cells of the intestinal epithelium of a tadpole and implanted them into egg cells whose nucleus he had removed. The egg cells developed (in the majority of cases) into fully functional adult frogs. Gurdon’s discoveries were initially met with huge scepticism because other researchers were unable to reproduce them. However, it eventually became apparent that this was because the researchers lacked the necessary dexterity and patience. Gurdon used microinjection experiments to elucidate which substances (DNA, RNA, proteins) in the plasma of egg cells are responsible for turning the cell nuclei back into their immature state. In the years after the discovery, Gurdon received many awards, was knighted and the institute at the University of Cambridge (sponsored by the Wellcome Trust/Cancer Research UK) where he has worked since 1972 was named the Gurdon Institute. He is still very active and was in his laboratory when he received the call from Stockholm.
Gurdon’s method of replacing the cell nucleus of egg cells with a nucleus from a mature, specialised cell was further developed, leading eventually to the cloning of animals (e.g., Dolly the sheep in 1997). However, the success rate is rather low.
Shinya Yamanaka was born in the same year that John Gurdon was carrying out his landmark nuclear transplantations. He initially trained as an orthopaedic surgeon before switching to basic research. Yamanaka was interested in finding out which genes kept stem cells – isolated from early mouse embryos and cultured in the laboratory – immature, i.e. pluripotent. Once Yamanaka and his co-workers had identified several such genes, they introduced them, in different combinations, into mature cells from connective tissue (fibroblasts). The genes were introduced into the cells with retroviruses.
Yamanaka’s discovery was published in 2006 and was immediately considered a major breakthrough. In this hallmark publication, Yamanaka reported that he and his co-workers were able to reprogramme fibroblasts into immature stems cells by introducing four genes (Oct4, Sox2, c-Myc and Klf4) together. The resulting stem cells were able to develop into mature cell types such as fibroblasts, nerve cells and gut cells. Yamanaka referred to the reprogrammed cells as induced pluripotent stem (iPS) cells. Intensive research in many laboratories around the world has since shown that iPS cells can develop into any kind of cell. iPS cells can also be produced from human cells.
The protocols for producing iPS cells without the need for embryos have since been refined and simplified. The functions of the genes that keep the cells in an immature state – transcription factors – have been subjected to in-depth analysis and considerable progress has been achieved. Recently, researchers even succeeded in removing the retrovirus from the iPS DNA after the genes had been transferred.
Hope for transplantation and cancer medicine

How realistic is the dream of curing people with stem cells, is the question Professor Dr. Andreas Trumpp, stem cell researcher at the German Cancer Research Center in Heidelberg, was asked in a TV interview (German ARD network) when the news broke from Stockholm. Trumpp said he was fairly optimistic about the prospects of success: “Yamanaka is relatively young for a Nobel Prize winner, and we can reasonably assume that the methods he has developed will soon contribute to helping people.”
Yamanaka’s findings were immediately recognised as highly important for cancer research. In November 2007, only a year after Yamanaka’s groundbreaking publication, a symposium was organised by the German Cancer Research Center in tribute to Yamanaka and he was awarded the Meyenburg Prize, one of the best funded scientific awards in Germany, for his outstanding achievements in cancer research.
Great hopes were placed on Yamanaka’s iPS cells, especially in Germany, where the ethical debate revolving around the use of human embryonic stem cells (hES cells) is particularly fierce. iPS cells do not have the same ethical drawbacks as hES cells as they can theoretically be obtained from the somatic cells of any human individual. This implies that every human individual could be the donor and recipient of his or her own stem cells, Trumpp explained. These stem cells could then be cultivated in the laboratory and grown into organs that would not be rejected by the recipient’s immune system. This would truly revolutionise the field of transplantation medicine.
However, all leading stem cell researchers agree that stem cell research cannot do without hES cells. These cells therefore remain the gold standard to which iPS cells need to measure up as their reprogramming is as yet far from perfect. The somatic cells from which iPS cells are produced accumulate mutations over time, for example as a result of environmental influences such as the UV radiation of the sun that affects skin cells. If stem cells are produced from such cells with the objective of using them for regenerative therapies, the modifications are also transferred and might lead to defects and even cancer. Trumpp explains: “Research in future will also need to involve both types of cells, embryonic stem cells and iPS cells.”

iPS cells will be used for research into human diseases and for the screening of drugs before effective stem cell therapies for the treatment of human diseases on the basis of reprogrammed somatic cells becomes available. Yamanaka’s discovery has created new ways to isolate iPS cells from healthy and sick individuals and allow them to differentiate into the tissue cells that researchers are seeking to investigate, such as nerve, heart and liver cells. These cells can then be used in the laboratory to study disease mechanisms and the effect of drugs.

![heralding-a-new-era-of-rna-therapeutics-358345-960x540[1]](https://blitzage.com/site/wp-content/uploads/2024/08/heralding-a-new-era-of-rna-therapeutics-358345-960x5401-1-300x169.jpg)