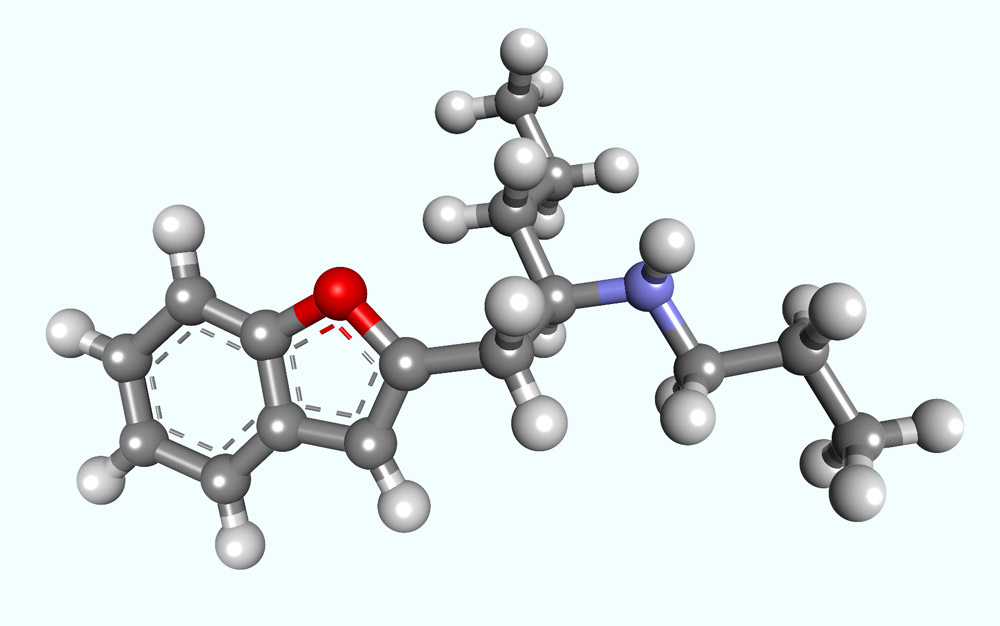
Antiaging Compounds: (‐)Deprenyl (Selegiline) and (‐)1‐(Benzofuran‐2‐yl)‐2‐propylaminopentane, [(‐)BPAP], a Selective Highly Potent Enhancer of the Impulse Propagation Mediated Release of Catecholamines and Serotonin in the Brain
Abstract
Hundreds of millions of people now die over the age of 80 years primarily due to twentieth century progress in hygiene, chemotherapy, and immunology. With a longer average lifespan, the need to improve quality of life during the latter decades is more compelling. “Aging — The Epidemic of the New Millenium,” a recent international conference (Monte Carlo, June 17–18, 2000), showed with peculiar clarity that a safe and efficient drug strategy to slow the age‐related decay of brain performance is still missing. This review summarizes the physiologic and pharmacologic arguments in favor of a peculiar lifelong prophylactic medication with reasonable chances to keep in check brain aging and decrease the precipitation of age‐related neurological diseases.
SUMMARY AND CONCLUSIONS
The specific brain activation mechanism (“drive”) that ensures that living beings surmount every obstacle to reach a goal, even if life is in the balance, roots in the existence of “enhancer‐sensitive” neurons in the brain that are ready to increase their activity with lightning speed in response to endogenous “enhancer” substances, of which phenylethylamine (PEA) and tryptamine are the presently known examples. PEA and tryptamine enhance the impulse‐propagation‐mediated release of catecholamines and serotonin in the brain (CAE/SAE effect). This is the best model for studying the enhancer regulation in the mammalian brain, which starts working at the discontinuation of breast feeding. Weaning is the beginning of the developmental (“uphill”) period of life and is characterized by significantly higher brain activity levels that last until the sexual hormones dampen this regulation, thereby terminating the uphill period. This is the prelude of the postdevelopmental (“downhill”) phase of life and the beginning of the slow brain aging process from which there is no escape until natural death.
It has been proposed that enhancer compounds can delay the natural age‐related deterioration of brain performance and keep the brain on a higher activity level during postdevelopment longevity. PEA, a substrate of MAO‐B, and tryptamine, a substrate of MAO‐A, are rapidly metabolized, short‐acting endogenous enhancer compounds. PEA and its long‐acting derivatives, amphetamine and methamphetamine, which are not metabolized by MAO, are enhancer substances at low concentrations but also potent releasers of catecholamines and serotonin from their pools at higher concentrations. The catecholamine‐releasing effect masked for decades the enhancer property of these compounds.
(‐)Deprenyl (selegiline) is the first PEA derivative free of the catecholamine‐releasing property and made possible the discovery of the enhancer regulation in the brain. This drug is presently the only clinically used enhancer compound. (‐)Deprenyl is also a highly potent, selective inhibitor of MAO‐B and is metabolized to amphetamines. Tryptamine is an endogenous enhancer substance free of the catecholamine/serotonin‐releasing property. The newly developed tryptamine derivative (‐)BPAP is the first highly selective enhancer substance. It is also much more potent than (‐)deprenyl.
Enhancer substances that keep the enhancer‐sensitive neurons on a higher activity level slow the age‐related deterioration of the mammalian brain. Maintenance of rats on (‐)deprenyl during post‐developmental longevity slows the age‐related decline of sexual and learning performances and prolongs life significantly. Patients with early Parkinson’s disease who are maintained on (‐)deprenyl need levodopa significantly later than their placebo‐treated peers and they live significantly longer when on levodopa plus (‐)deprenyl than patients on levodopa alone. In patients with moderately severe impairment from Alzheimer’s disease, treatment with (‐)deprenyl slows the progression of the disease.
(‐)BPAP is an especially promising prophylactic antiaging compound that may provide the opportunity to shift the functional constellation of the brain during postdevelopmental longevity towards the one characteristic to the uphill period of life. According to the available experimental and clinical data, it is reasonable to expect that daily administration of an enhancer drug [e.g., (‐)deprenyl 1 mg or (‐)BPAP 0.1 mg] from sexual maturity until death will improve quality of life in the latter decades, shift the time of natural death, decrease the precipitation of age‐related depression, and reduce the prevalence of Parkinson’s disease and Alzheimer’s disease.
Keywords: Selegiline, Deprenyl, Antiaging drugs, BPAP
Full Text
The Full Text of this article is available as a PDF (542K).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
1. Agnoli A, Fabbrini G, Fioravanti M, Martucci N. CBF and cognitive evaluation of Alzheimer‐type patients before and after MAO‐B treatment: A pilot study. Eur Neuropsychopharmacol 1992;2:31–35. [PubMed] [Google Scholar]
2. Allain H, Gougnard J, Naukirek HC. Selegiline in de novo parkinsonian patients: The French selegiline multicenter trial (FSMP). Acta Neurol Scand 1991;136:73–78. [PubMed] [Google Scholar]
3. Archer JR, Harrison DE. L‐Deprenyl treatment in aged mice slightly increases lifespans and greatly reduces fecundity by aged males. J Gerontol Sci 1996;13A:B448–B453. [PubMed] [Google Scholar]
4. Birkmayer W, Hornykiewicz O. Der L‐Dioxyphenyl‐alanin‐Effekt beim Parkinson Syndrom des Menschen. Arch Psychiat Nervenkrh 1962;203:560–564. [Google Scholar]
5. Birkmayer W, Riederer P. Parkinson’s Disease. Biochemistry, Clinical Pathology and Treatment. New York : Springer‐Verlag, 1983:1–194. [Google Scholar]
6. Birkmayer W, Riederer P, Ambrozi L, Youdim MBH. Implications of combined treatment with “Madopar” and L‐Deprenil in Parkinson’s disease. Lancet 1977;1:439–443. [PubMed] [Google Scholar]
7. Birkmayer W, Riederer P, Linauer W, Knoll J. L‐Deprenyl plus L‐phenylalanine in the treatment of depression. J Neural Transm 1984;59:81–87. [PubMed] [Google Scholar]
8. Birkmayer W, Knoll J, Riederer P, Youdim MBH, Hars V, Marton J. Increased life expectancy resulting from addition of L‐deprenyl to Madopar treatment in Parkinson’s disease: A long‐term study. J Neural Transm 1985;64:113–127. [PubMed] [Google Scholar]
9. Burke WJ, Roccaforte WH, Wengel SP, Bayer BL, Ranno AE, Willcockson NK. L‐Deprenyl in the treatment of mild dementia of the Alzheimer type: Results of a 15‐month trial. J Am Geriatr Soc 1993;41:1219–1225. [PubMed] [Google Scholar]
10. Campi N, Todeschini GP, Scarzella L. Selegiline versus L‐acetylcarnitine in the treatment of Alzheimer‐type dementia. Clin Ther 1990;12:306–314. [PubMed] [Google Scholar]
11. Carrillo MC, Kanai S, Nokubo M, Kitani K. (‐)Deprenyl induces activities of both superoxide dismutase and catalase but not of glutathion peroxidase in the striatum of young male rats. Life Sci 1991;48:517–521. [PubMed] [Google Scholar]
12. Carrillo MC, Kanai S, Nokubo M, Ivy GO, Sato Y, Kitani K. (‐)Deprenyl increases activities of superoxide dismutase and catalase in striatum but not in hippocampus: The sex‐ and age‐related differences in the optimal dose in the rat. Exp Neurol 1992;116:286–294. [PubMed] [Google Scholar]
13. Cohen G, Pasik P, Cohen B, Leist A, Mitileneou C, Yahr MD. Pargyline and (‐)deprenyl prevent the neurotoxicity of 1‐methyl‐4‐phenyl‐1,2,3,6‐tetra‐hydropyridine (MPTP) in monkeys. Eur J Pharmacol 1984;106:209–210. [PubMed] [Google Scholar]
14. Elsworth JD, Glover V, Reynolds GP, et al. Deprenyl administration in man: A selective monoamine oxidase B inhibitor without the “cheese effect. Psychopharmacology 1978;57:33–38. [PubMed] [Google Scholar]
15. Falsaperle A, Monici Preti PA, Oliani C. Selegiline versus oxiracetam in patients with Alzheimer‐type dementia. Clin Ther 1990;12:376–384. [PubMed] [Google Scholar]
16. Finali G, Piccirilli M, Oliani C, Piccinin GL. Alzheimer‐type dementia and verbal memory performances: Influence of selegiline therapy. Ital J Neurol Sci 1992;13:141–148. [PubMed] [Google Scholar]
17. Finali G, Piccirilli M, Oliani C, Piccinin GL. L‐deprenyl therapy improves verbal memory in amnesic Alzheimer patients. Clin Neuropharmacol 1991;14:523–536. [PubMed] [Google Scholar]
18. Finnegan KT, Skratt JJ, Irvin I, DeLanney LE, Langston JW. Protection against DSP‐4 induced neurotoxicity by deprenyl is not related to its inhibition of MAO‐B. Eur J Pharmacol 1990;184:119–126. [PubMed] [Google Scholar]
19. Gallagher IM, Clow A, Glover V. Long term administration of (‐)deprenyl increases mortality in male Wistar rats. J Neural Transm 1998;52 (Suppl): 315–320. [PubMed] [Google Scholar]
20. Goad DL, Davis CM, Liem P, Fuselier CC, McCormack JR, Olsen KM. The use of selegiline in Alzheimer’s patients with behavior problems. J Clin Psychiatry 1991;52:342–345. [PubMed] [Google Scholar]
21. Groc L, Levine RA, Foster JA, Normile HJ, Weissmann D, Bezin L. Evidence of deprenyl‐insensitive apoptosis of nigral dopamine neurons during development. Brain Res Dev Brain Res 2000;120:95–98. [PubMed] [Google Scholar]
22. Hársing RG, Magyar K, Tekes K, Vizi ES, Knoll J. Inhibition by (‐)‐deprenyl of dopamine uptake in rat striatum: A possible correlation between dopamine uptake and acetylcholine release inhibition. Pol J Pharmacol Pharm 1979;31:297–307. [PubMed] [Google Scholar]
23. Ingram DK, Wiener HL, Chachich ME, Longo JM, Hengemihle J, Gupta M. Chronic treatment of aged mice with L‐deprenyl produced marked MAO‐B inhibition but no beneficial effects on survival, motor performance, or nigral lipofuscin accumulation. Neurobiol Aging 1993;14:431–440. [PubMed] [Google Scholar]
24. Kitani K, Kanai S, Sato Y, Ohta M, Ivy GO, Carillo MC. Chronic treatment of (‐)deprenyl prolongs the lifespan of male Fischer 344 rats. Further evidence. Life Sci 1992;52:281–288. [PubMed] [Google Scholar]
25. Knoll J. Experimental studies on the higher nervous activity of animals. VI. Further studies on active reflexes. Acta Physiol Hung 1957;12:65–92. [PubMed] [Google Scholar]
26. Knoll J. The Theory of Active Reflexes. An Analysis of Some Fundamental Mechanisms of Higher Nervous Activity. New York : Hafner Publishing Company, 1969:1–131. [Google Scholar]
27. Knoll J. Analysis of the pharmacological effects of selective monoamine oxidase inhibitors In: Monoamine Oxidase and Its Inhibition. Wolstenholme GES, Knight J, Eds. Amsterdam : Elsevier, 1976:131–161. [Google Scholar]
28. Knoll J. The possible mechanism of action of (‐)deprenyl in Parkinson’s disease. J Neural Transm 1978;43:177–198. [PubMed] [Google Scholar]
29. Knoll J. Selective inhibition of B type monoamine oxidase in the brain: A drug strategy to improve the quality of life in senescence In: Strategy in Drug Research. Keverling Buisman JA, ed. Amsterdam : Elsevier, 1982:107–135. [Google Scholar]
30. Knoll J. Deprenyl (selegiline). The history of its development and pharmacological action. Acta Neurol Scand 1983;59 (Suppl): 57–80. [PubMed] [Google Scholar]
31. Knoll J. The striatal dopamine dependency of lifespan in male rats. Longevity study with (‐)deprenyl. Mech Ageing Dev 1988;46:237–262. [PubMed] [Google Scholar]
32. Fadda P, Scherma M, Fresu A, Coliu M, Fratta W. Baclofen antagonizes nicotine‐, cocaine‐, and morphineinduced dopaminie release in the nucleus accumbens of rat. Synapse 2003;50:1–6. [PubMed] [Google Scholar]
33. Knoll J. Nigrostriatal dopaminergic activity, deprenyl treatment, and longevity. Adv Neurol 1990;53:425–429. [PubMed] [Google Scholar]
34. Knoll J. Pharmacological basis of the therapeutic effect of (‐)deprenyl in age‐related neurological diseases. Med Res Rev 1992;12:505–524. [PubMed] [Google Scholar]
35. Knoll J. Memories of my 45 years in research. Pharmacol Toxicol 1994;75:65–72. [PubMed] [Google Scholar]
36. Knoll J. Rationale for (‐)deprenyl (selegiline) medication in Parkinson’s disease and in prevention of age‐related nigral changes. Biomed Pharmacother 1995;49:187–195. [PubMed] [Google Scholar]
37. Knoll J. (‐)Deprenyl (selegiline), a catecholaminergic activity enhancer (CAE) substance acting in the brain. Pharmacol Toxicol 1998;82:57–66. [PubMed] [Google Scholar]
38. Knoll J, Magyar K. Some puzzling effects of monoamine oxidase inhibitors. Adv Bioch Psychopharmacol 1972;5:393–408. [PubMed] [Google Scholar]
39. Knoll J, Miklya I. Enhanced catecholaminergic and serotoninergic activity in rat brain from weaning to sexual maturity. Rationale for prophylactic (‐)deprenyl (selegiline) medication. Life Sci 1995;56:611–620. [PubMed] [Google Scholar]
40. Knoll J, Ecseri Z, Kelemen K, Nievel J, Knoll B. Phenylisopropylmethyl‐ propinylamine (E‐250), a new psychic energizer. Arch Int Pharmacodyn Ther 1965;155:154–164. [PubMed] [Google Scholar]
41. Knoll J, Vizi ES, Somogyi G. Phenylisopropylmethylpropinylamine (E‐250), a monoamine oxidase inhibitor antagonizing the effects of tyramine. Arzneimittelforschung 1968;18:109–112. [Google Scholar]
42. Knoll J, Yen TT, Dalló J. Long‐lasting, true aphrodisiac effect of (‐)deprenyl in sexually sluggish old male rats. Mod Prob Pharmacopsychiatry 1983;19:135–153. [PubMed] [Google Scholar]
43. Knoll J, Dalló J, Yen TT. Striatal dopamine, sexual activity and lifespan. Longevity of rats treated with (‐)deprenyl. Life Sci 1989;45:525–531. [PubMed] [Google Scholar]
44. Knoll J, Knoll B, Török Z, Timár J, Yasar S. The pharmacology of 1‐phenyl‐2‐propylaminopentane (PPAP), a deprenyl‐derived new spectrum psychostimulant. Arch Int Pharmacodyn Ther 1992;316:5–29. [PubMed] [Google Scholar]
45. Knoll J, Tóth V, Kummert M, Sugár J. (‐)Deprenyl and (‐)parafluorodeprenyl‐ treatment prevents age‐related pigment changes in the substantia nigra. A TV‐image analysis of neuromelanin. Mech Ageing Dev 1992;63:157–163. [PubMed] [Google Scholar]
46. Knoll J, Yen TT, Miklya I. Sexually low performing male rats die earlier than their high performing peers and (‐)deprenyl treatment eliminates this difference. Life Sci 1994;54:1047–1057. [PubMed] [Google Scholar]
47. Knoll J, Miklya I, Knoll B, Markó R, Kelemen K. (‐)Deprenyl and (‐)1‐phenyl‐2‐propylaminopentane, [(‐)PPAP], act primarily as potent stimulants of action potential‐transmitter release coupling in the catecholaminergic neurons. Life Sci 1996;58:817–827. [PubMed] [Google Scholar]
48. Knoll J, Miklya I, Knoll B, Markó R, Rácz D. Phenylethylamine and tyramine are mixed‐acting sympathomimetic amines in the brain. Life Sci 1996;58:2101–2114. [PubMed] [Google Scholar]
49. Knoll J, Yoneda F, Knoll B, Ohde H, Miklya I. (‐)1‐(Benzofuran‐2‐yl)‐2‐propylaminopentane, [(‐)BPAP], a selective enhancer of the impulse propagation mediated release of catecholamines and serotonin in the brain. Br J Pharmacol 1999;128:1723–1732. [PMC free article] [PubMed] [Google Scholar]
50. Knoflach F, Mutel V, Jolidon S, et al. Positive allosteric modulators of metabotropic glutamate 1 receptor: Characterization, mechanism of action, and binding site. Proc Natl Acad Sci USA 2001;98:13402–13407. [PMC free article] [PubMed] [Google Scholar]
51. Larsen JP, Boas J, Erdal JE. Does selegiline modify the progression of early Parkinson’s disease? Results from a five‐year study. The Norwegian‐Danish Study Group. Eur J Neurol 1999;6:539–547. [PubMed] [Google Scholar]
52. Lees AJ. Selegiline hydrochloride and cognition. Acta Neurol Scand 1991;136 (Suppl): 91–94. [PubMed] [Google Scholar]
53. Lees AJ. Comparison of therapeutic effects and mortality data of levodopa and levodopa combined with selegiline in patients with early, mild Parkinson’s disease. Br Med J 1995;311:1602–1607. [PMC free article] [PubMed] [Google Scholar]
54. Mangoni A, Grassi MP, Frattola L, Piolti R, Bassi S, Motta A. Effects of a MAO‐B inhibitor in the treatment of Alzheimer disease. Eur Neurol 1991;31:100–107. [PubMed] [Google Scholar]
55. Mann JJ, Gershon S. A selective monoamine oxidase‐B inhibitor in endogenous depression. Life Sci 1980;26:877–882. [PubMed] [Google Scholar]
56. Martin C. Sexual activity in the aging male In: Handbook of Sexology. Money J, Musaph H, Eds. Amsterdam : Elsevier, 1977:813–824. [Google Scholar]
57. Martini E, Pataky I, Szilágyi K, Venter V. Brief information on an early phase‐II study with (‐)deprenyl in demented patients. Pharmacopsychiatry 1987;20:256–257. [PubMed] [Google Scholar]
58. Milgram MW, Racine RJ, Nellis P, Mendoca A, Ivy GO. Maintenance on L‐(‐)deprenyl prolongs life in aged male rats. Life Sci 1990;47:415–420. [PubMed] [Google Scholar]
59. Monteverde A, Gnemmi P, Rossi F, Monteverde A, Finali GC. Selegiline in the treatment of mild to moderate Alzheimer‐type dementia. Clin Ther 1990;12:315–322. [PubMed] [Google Scholar]
60. Myttyla VV, Sotaniemi KA, Vourinen JA, Heinonen EH. Selegiline as initial treatment in de novo parkinsonian patients. Neurology 1992;42:339–343. [PubMed] [Google Scholar]
61. . Parkinson Study Group. Effect of (‐)deprenyl on the progression disability in early Parkinson’s disease. New Engl J Med 1989;321:1364–1371. [PubMed] [Google Scholar]
62. . Parkinson Study Group. Effect to tocopherol and (‐)deprenyl on the progression of disability in early Parkinson’s disease. New Engl J Med 1993;328:176–183. [PubMed] [Google Scholar]
63. . Parkinson Study Group. Impact of deprenyl and tocopherol treatment of Parkinson’s disease in DATATOP patients requiring levodopa. Ann Neurol 1996;39:37–45. [PubMed] [Google Scholar]
64. Phillips SL. Amphetamine, p‐hydroxyamphetamine and β‐phenylethylamine in mouse brain and urine after (‐)‐ and (+)‐deprenyl administration. J Pharm Pharmacol 1981;31:739–741. [PubMed] [Google Scholar]
65. Piccinin GL, Finali GC, Piccirilli M. Neuropsychological effects of L‐deprenyl in Alzheimer’s type dementia. Clin Neuropharmacol 1990;13:147–163. [PubMed] [Google Scholar]
66. Sandler M, Glover V, Ashford A, Stern GM. Absence of “cheese effect” during deprenyl therapy: Some recent studies. J Neural Transm 1978;43:209–215. [PubMed] [Google Scholar]
67. Reynolds GP, Elsworth JD, Blau K, Sandler M, Lees AJ, Stern GM. Deprenyl is metabolized to methamphetamine and amphetamine in man. Br J Clin Pharmacol 1978;6:542–544. [PMC free article] [PubMed] [Google Scholar]
68. Rinne JO, Röyttä M, Paljärvi L, Rummukainen J, Rinne UK. Selegiline (deprenyl) treatment and death of nigral neurons in Parkinson’s disease. Neurology 1991;41:859–861. [PubMed] [Google Scholar]
69. Ruehl WW, Entriken TL, Muggenberg BA, Bruyette DS, Griffith WG, Hahn FF. Treatment with L‐deprenyl prolongs life in elderly dogs. Life Sci 1997;61:1037–1044. [PubMed] [Google Scholar]
70. Sano M, Ernesto C, Klauber MR, and Members of the Alzheimer’s disease Cooperative Study. Rationale and design of a multicenter study of selegiline and α‐tocopherol in the treatment of Alzheimer disease using novel clinical outcomes. Alzheimer Dis Assoc Disord 1996;10:132–140. [PubMed] [Google Scholar]
71. Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha‐tocopherol, or both as treatment for Alzheimer’s disease. New Engl J Med 1997;336:1216–1222. [PubMed] [Google Scholar]
72. Schneider LS, Pollock VE, Zemansky MF, Gleason RP, Palmer R, Sloane RB. A pilot study of low‐dose L‐deprenyl in Alzheimer’s disease. J Geriatr Psychiatry Neurol 1991;4:143–148. [PubMed] [Google Scholar]
73. Stoll S, Hafner U, Kranzlin B, Muller WE. Chronic treatment of Syrian hamsters with low‐dose selegiline increases lifespan in females but not males. Neurobiol Aging 1997;18:205–211. [PubMed] [Google Scholar]
74. Suuronen T, Kolehmainen P, Salminen A. Protective effect of L‐deprenyl against apoptosis induced by okadaic acid in cultured neuronal cells. Biochem Pharmacol 2000;59:1589–1595. [PubMed] [Google Scholar]
75. Tariot PN, Cohen RM, Sunderland T, Newhouse PA, Yount D, Mellow AM. L‐(‐)deprenyl in Alzheimer’s disease. Arch Gen Psychiatry 1987;44:427–433. [PubMed] [Google Scholar]
76. Tatton WG. Apoptotic mechanisms in neurodegeneration: Possible relevance to glaucoma. Eur J Ophthal mol 1999;9 (Suppl): S22–S29. [PubMed] [Google Scholar]
77. Tetrud JW, Langston JW. The effect of (‐)deprenyl (selegiline) on the natural history of Parkinson’s disease. Science 1989;245:519–522. [PubMed] [Google Scholar]
78. Tringer L, Haits G, Varga E. The effect of L‐E‐250 (‐L‐phenyl‐isopropylmethyl‐propinyl‐amine HCl) in depression In: V. Conferentia Hungarica pro Therapia et Investigatione in Pharmacologia. Leszkovszky G, Ed. Budapest : Publishing House of the Hungarian Academy of Sciences, 1971:111–114. [Google Scholar]
79. ThyagaRajan S, Meites J, Quadri SK. Deprenyl reinitiates estrous cycles, reduces serum prolactin, and decrease the incidence of mammary and pituitary tumors in old acyclic rats. Endocrinology 1995;136:1103–1110. [PubMed] [Google Scholar]
80. ThyagaRajan S, Felten SY, Felten DL. Antitumor effct of L‐deprenyl in rats with carcinogen‐induced mammary tumors. Cancer Lett 1998;123:177–183. [PubMed] [Google Scholar]
81. Varga E. Vorläufiger Bericht über die Wirkung des Präparates E‐250 (phenyl‐ isopropyl‐methyl‐propinyl‐amine‐chlorhydrat) In: III Conferentia Hungarica pro Therapia et Investigatione in Pharmacologia. Dumbovich B, ed. Budapest : Publishing House of the Hungarian Academy of Sciences, 1965:197–201. [Google Scholar]
82. Varga E, Tringer L. Clinical trial of a new type of promptly acting psychoenergetic agent (phenyl‐isopropyl‐methyl‐propinylamine HCl, E‐250). Acta Med Acad Sci Hung 1967;23:289–295. [PubMed] [Google Scholar]
83. Vizuete ML, Steffen V, Ayala A, Cano J, Machado A. Protective effect of deprenyl against 1‐methyl‐4‐phenylpyridinium neurotoxicity in rat striatum. Neurosci Lett 1993;152:113–116. [PubMed] [Google Scholar]
84. Wu RM, Chiuech CC, Pert A, Murphy DL. Apparent antioxidant effect of l‐deprenyl on hydroxyl radical formation and nigral injury elicited by MPP+ in vivo. Eur J Pharmacol 1993;243:241–247. [PubMed] [Google Scholar]
85. Yasar S, Winger G, Nickel B, Schulze G, Goldberg SR. Preclinical evaluation of l‐deprenyl: Lack of amphetamine‐like abuse potential In: Inhibitors of Monoamine Oxidase B. Szelenyi I, Ed. Basel : Birkhäuser Verlag, 1993:215–233. [Google Scholar]
86. Yen TT, Knoll J. Extension of lifespan in mice treated with Dinh lang (Policias fruticosum L) and (‐)deprenyl. Acta Physiol Hung 1992;79:119–124. [PubMed] [Google Scholar]




