
Autophagy (self‐eating) is a conserved catabolic homeostatic process required for cellular metabolic demands by removal of the damaged molecules and organelles and for alleviation of stress initiated by pathology and infection. By such actions, autophagy is essential for the prevention of aging, disease, and cancer. Genetic defects of autophagy genes lead to a host of developmental, metabolic, and pathological aberrations. Similarly, the age‐induced decline in autophagy leads to the loss of cellular homeostatic control. Paradoxically, such a valuable mechanism is hijacked by diseases, during tumor progression and by senescence, presumably due to high levels of metabolic demand. Here, we review both the role of autophagy in preventing cellular decline in aging by fulfillment of cellular bioenergetic demands and its contribution to the maintenance of the senescent state and SASP by acting on energy and nutritional sensors and diverse signaling pathways.
Various forms of autophagy. Major types of autophagy include macroautophagy, microautophagy (inside the circle), and chaperone‐mediated autophagy. Autophagy is used for the removal of damaged biomolecules and organelles (outside the circle).
The term “autophagy” existed from the middle of the 19th century and was coined in 1963 by the Nobel Laurette, Christian René Marie Joseph, Viscount de Duve, after discovery of lysosomes (Porter & Novikoff, 1974). Since then, the intricate nature of the process has come to light. It is shown that autophagy is required for fulfillment of cellular metabolic demands, preservation of genomic integrity, innate, and adaptive immune processes, regulation of pro‐inflammatory mediators and for forstering the cell survival. Autophagy is evolutionarily conserved and serves as a ubiquitous, self‐degradative catabolic process that removes long lived, damaged molecules and organelles, aggregated proteins, and intracellular pathogens.
Basal autophagy occurs constitutively in nutrient‐rich environments for the turnover of the cellular components as a fundamental survival mechanism. However, the induced or reactive autophagy is engaged by stresses such as starvation, low level of amino acids, trophic factor or hormone deprivation, heat, and ER stress, hypoxia, irradiation, drugs, and intracellular pathogens (Komatsu et al., 2007). Major types of autophagy include macroautophagy, microautophagy, and chaperone‐mediated autophagy (CMA). Autophagy is also used for the removal of damaged organelles including mitochondria (mitophagy), ER (ER‐phagy), lysosome (lysophagy), among others and for the removal of damaged macromolecules and foreign pathogens (Figure 1) (Jia et al., 2020).

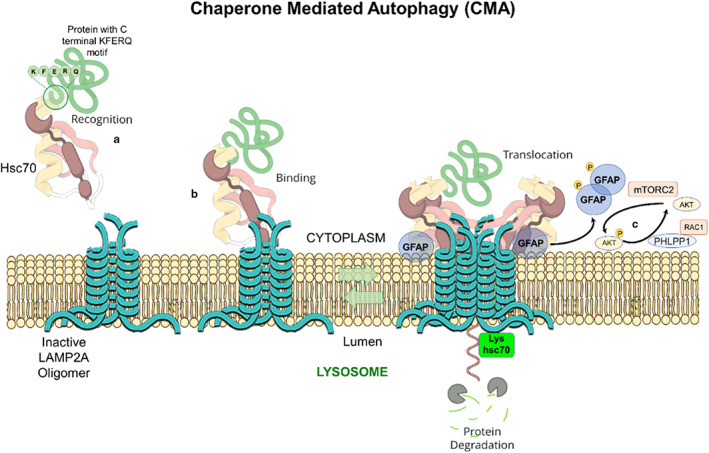
Macroautophagy applies for the bulk and non‐selective removal of macromolecules or subcellular organelles within cytosolic double‐membrane‐bound autophagosomes fused with lysosomes (Figure 1) (Mijaljica et al., 2011; Feng et al., 2014; Schütter et al., 2020; Aman et al., 2021; Yim & Mizushima, 2020). Microautophagy and micro‐ERphagy can occur in a non‐selective way for the sequestration of the bulk of cytosolic substrates under starvation conditions or can occur with high precision by allowing specific cargoes to be captured before they enter the invaginations of endolysosome membranes and get digested by lysosomes (Figure 1) (Hansen et al., 2018). Chaperone‐mediated autophagy (CMA), which is induced after long‐term starvation, uses the chaperone, heat shock protein 70 (HSC70; 71‐kDa, also known as HSPA8) for interaction with cytosolic proteins with its C‐terminal KFERQ pentapeptide sequence (Figure 2) (Alfaro et al., 2019, Mizushima et al., 2008). Autophagy uses cargo receptors and uses the interaction with LC3 in autophagic membranes, to specifically remove organelles (Seglen et al., 1990, Zaffagnini and Martens, 2016) (Figure 1).
Protein aggregates are removed by a type of macroautophagy called aggrephagy (Hyttinen et al., 2014, Kaushik and Cuervo 2018). DNA and RNA are bound in an ATP‐dependent and CMA‐like manner (Fujiwara et al., 2015, Aizawa et al., 2017). Glycophagy is a hormonally controlled and highly regulated process, requiring many signaling pathways such as the cyclic AMP protein kinase A/protein kinase A, PI3K‐Akt/PKB‐mTOR, and calcium (Zhao et al., 2018). Lipophagy is required for maintaining the energy balance by the breakdown of lipids. Lipophagy requires the participation of autophagy, by recruiting a complex network of over 32 proteins of autophagy and autophagy‐related proteins. (Greenberg et al., 2011, Fujimoto and Parton, 2011, Singh and Cuervo, 2012). Granulophagy removes the stress granules and P bodies (Buchan et al., 2013). Mitochondrial integrity is maintained and removal of damaged mitochondria becomes feasible in vivo, by the joint participation of two Parkinson’s disease genes, mitochondrial kinase, PINK1, and ubiquitin ligase, Parkin. Triggering mitophagy requires PINK1 phosphorylation of Parkin and ubiquitin with USP30 deubiquitinase, which acts as a brake in this process, by opposing Parkin‐mediated ubiquitination. Mitophagy allows damaged mitochondria to be captured by binding the soluble or membrane‐bound mitophagy receptors (mReceptors), followed by binding LC3, recruitment of p62 before engulfment, formation of autophagosomes, and their subsequent delivery to lysosomes for degradation (Onishi et al., 2021, Bingol and Sheng, 2016, Abudu et al., 2021). The term, “MitophAging” is suggested to include age‐induced diseases that result from loss of mitophagy (Bakula and Scheibye‐Knudsen, 2020). Damaged ER fragmentation requires atlastin 2 (ATL2), a GTPase‐mediating homotypic fusion of the ER. Some ER domains, utilize Atg8, whereas certain ER‐specific domains use other types of receptors (Dikic and Elazar, 2018, Stolz and Grumati, 2019).
There is increasing evidence that aging leads to the loss of expression of autophagy genes and substantially reduces the activity of selective as well as non‐selective autophagy (Leidal et al., 2018, Simonsen et al., 2008). Forced genetic impairment of autophagy induces an accelerated decline in cellular functions whereas an increase in autophagy delays aging in animal models (Leidal et al., 2018, Hansen et al., 2018). Aging also results in the generation of oxysterols such as 7‐ketocholesterol‐ and 7β‐hydroxycholesterol. These oxysterols result in the dysfunction of mitochondria and peroxisomes, oxidative stress, and a type of autophagic cell death, called oxiapoptophagy (Nury et al, 2021). Here, we briefly summarize various forms of autophagy.




