Abstract
Senotherapy is an antiageing strategy. It refers to selective killing of senescent cells by senolytic agents, strengthening the activity of immune cells that eliminate senescent cells or alleviating the secretory phenotype (SASP) of senescent cells. As senescent cells accumulate with age and are considered to be at the root of age-related disorders, senotherapy seems to be very promising in improving healthspan. Genetic approaches, which allowed to selectively induce death of senescent cells in transgenic mice, provided proof-of-concept evidence that elimination of senescent cells can be a therapeutic approach for treating many age-related diseases. Translating these results into humans is based on searching for synthetic and natural compounds, which are able to exert such beneficial effects. The major challenge in the field is to show efficacy, safety and tolerability of senotherapy in humans. The question is how these therapeutics can influence senescence of non-dividing post-mitotic cells. Another issue concerns senescence of cancer cells induced during therapy as there is a risk of resumption of senescent cell division that could terminate in cancer renewal. Thus, development of an effective senotherapeutic strategy is also an urgent issue in cancer treatment. Different aspects, both beneficial and potentially detrimental, will be discussed in this review.
Introduction
In the modern world the population of very old people is increasing and we have realized that successful intervention in the lifespan is possible. A desirable outcome of such intervention would be ageing free of age-related diseases, such as metabolic and cardiovascular disease, neurodegeneration, cancer and many others. Geroscience aims to understand the relationship between ageing, chronic age-related diseases (ARDs) and geriatric syndromes (GSs) and assumes that ageing and ARDs/GSs share a common set of basic biological mechanisms (Franceschi et al., 2018). It has been suggested that these mechanisms include cell senescence. Indeed, recently it has been proposed that elimination of senescent cells in genetically modified mice can rejuvenate the organism (Childs et al., 2015). The just coined term “senotherapy” refers to the process of body rejuvenation by pharmacological treatment with senolytic drugs selectively eradicating senescent cells (Sturmlechner et al., 2017; Zhu et al., 2015).
Section snippets
Cellular senescence
The term “cellular senescence”, introduced almost 60 years ago, referred to the limited capacity of cell population to divide in culture (Hayflick and Moorhead, 1961), which later on has been attributed to telomere shortening (Harley et al., 1990). Since then, it has been observed that not only telomere shortening, but also oncogenes and some sorts of stress were able to stop cell divisions and induce cell senescence. These phenomena were termed OIS (oncogene-induced senescence) (Kuilman et
Transgenic mouse models used in senotherapy
In recent years, several transgenic mouse models have been developed that make possible the visualization, assessment and eradication of senescent cells in vivo. In these models the promoter of p16INK4A gene was used to drive the expression of genes encoding proteins, which induce death of senescent cells upon administration of small molecules (transgene activator). Beside death-inducing product of the transgene also expression of fluorescent proteins can be regulated by p16INK4A promoter in
Senolytics in vitro and in vivo
Senescent cells are resistant to apoptosis. On the other hand some SASP factors are pro-apoptotic. Moreover, pro-apoptotic pathways were shown to be up-regulated in senescent cells (Zhu et al., 2015), To solve this paradox the hypothesis was tested that senescent cells depend on pro-survival pathways, which inhibit apoptosis, in order to defend themselves against their own pro-apoptotic SASP. Using bioinformatics approaches based on the RNA and protein expression profiles of senescent cells,
Senotherapy in alleviation of age-related diseases
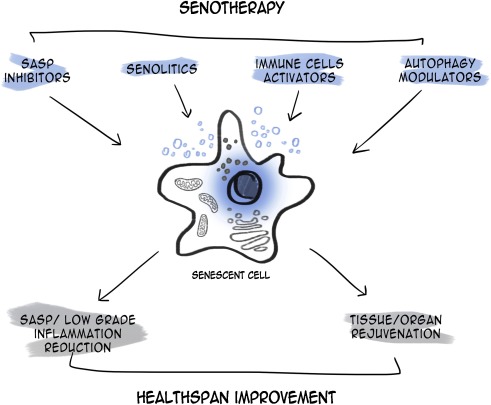
The results obtained in mouse transgenic models and upon treatment with senolytics give hope that many age-related diseases in humans can be cured. The potential outcome of senotherapy is outlined on Fig. 1 and detailed description of the results obtained in animal experiments, both genetic and pharmacological, are presented in Table 1. Their number is growing quite rapidly. Some of these results are discussed below.
Senotherapeutic approach to treat cancer and to alleviate side-effects of anticancer therapy
Increasing age is one of the strongest risk factors for cancer development. On the other hand, cell senescence is an important barrier to cancer development as it protects against spreading cells with harmful mutations.
At the end of the previous century Serrano and colleagues showed that in vitrooverexpression of the Ras oncogene in normal cells leads to senescence (OIS, oncogene induced senescence), while transfection of immortalized cells with the same oncogene facilitates cell transformation
Autophagy in senotherapy
Autophagy is a catabolic process, in which proteins, other macromolecules, and organelles are degraded and recycled, thus providing metabolites to maintain energy supply in the cell. It also prevents “waste” accumulation in a tissue. Three main types of autophagic processes have been described to date: macroautophagy, microautophagy, and chaperone-mediated autophagy (Gomez-Sintes et al., 2016). As macroautophagy has been the best recognized type of autophagy, the term autophagy usually refers
Questions and remarks
Pharmacological and nutraceutical elimination of senescent cells from the body seems to be very promising in postponing ageing and age-related diseases. However, results obtained so far originate mainly from animal and tissue culture studies, even though the first human pilot study has been successfully completed (Justice et al., 2019). Namely, fourteen patients with stable mild severe idiopathic pulmonary fibrosis (IPF) were treated with 9 oral doses of D + Q over 3 weeks. Physical function
Funding
This study was supported by National Science Centre grants: UMO-2016/21/B/NZ3/00370 (to ABZ), UMO 2014/15/B/NZ3/01150 (to GM) and UMO-2015/17/B/NZ3/03531(to ES)
Declaration of Competing Interest
The authors confirm no conflict of interest
Acknowledgements
The authors thanks Karolina Mosieniak for preparing the picture
References (168)
- S. Akunuru et al.Aging, clonality, and rejuvenation of hematopoietic stem cellsTrends Mol. Med.(2016)
- D. Bandyopadhyay et al.The human melanocyte: a model system to study the complexity of cellular aging and transformation in non-fibroblastic cellsExp. Gerontol.(2001)
- A. Bielak-Zmijewska et al.Is DNA damage indispensable for stress-induced senescence?Mech. Ageing Dev.(2018)
- A. Brzezinska et al.Proliferation of CD8+ in culture of human T cells derived from peripheral blood of adult donors and cord blood of newbornsMech. Ageing Dev.(2003)
- A. Brzezinska et al.Proliferation and apoptosis of human CD8(+)CD28(+) and CD8(+)CD28(-) lymphocytes during agingExp. Gerontol.(2004)
- A. Burkle et al.MARK-AGE biomarkers of ageingMech. Ageing Dev.(2015)
- D.G.A. Burton et al.Cellular senescence: immunosurveillance and future immunotherapyAgeing Res. Rev.(2018)
- S.J. Chinta et al.Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to parkinson’s diseaseCell Rep.(2018)
- W. Damsky et al.mTORC1 activation blocks BrafV600E-induced growth arrest but is insufficient for melanoma formationCancer Cell(2015)
- J.P. de Magalhaes et al.Stress, cell senescence and organismal ageingMech. Ageing Dev.(2018)
- M. Demaria et al.An essential role for senescent cells in optimal wound healing through secretion of PDGF-AADev. Cell(2014)
- R.B. Effros et al.In vitro senescence of immune cellsExp. Gerontol.(2003)
- Y.Q. Geng et al.Senescence-associated beta-galactosidase activity expression in aging hippocampal neuronsBiochem. Biophys. Res. Commun.(2010)
- R. Gomez-Sintes et al.Lysosomal cell death mechanisms in agingAgeing Res. Rev.(2016)
- F. Gurau et al.Anti-senescence compounds: a potential nutraceutical approach to healthy agingAgeing Res. Rev.(2018)
- D. Hanahan et al.The hallmarks of cancerCell(2000)
- L. Hayflick et al.The serial cultivation of human diploid cell strainsExp. Cell Res.(1961)
- J.C. Jeyapalan et al.Cellular senescence and organismal agingMech. Ageing Dev.(2008)
- J.N. Justice et al.Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot studyEBioMedicine(2019)
- D. Kashyap et al.Fisetin: a bioactive phytochemical with potential for cancer prevention and pharmacotherapyLife Sci.(2018)
- J.L. Kirkland et al.Cellular senescence: a translational perspectiveEBioMedicine(2017)
- V. Krizhanovsky et al.Senescence of activated stellate cells limits liver fibrosisCell(2008)
- T. Kuilman et al.Oncogene-induced senescence relayed by an interleukin-dependent inflammatory networkCell(2008)
- S. Mansilla et al.A nuclear budding mechanism in transiently arrested cells generates drug-sensitive and drug-resistant cellsBiochem. Pharmacol.(2009)
- R. Anderson et al.Length-independent telomere damage drives post-mitotic cardiomyocyte senescenceEMBO J(2019)
- M.P. Baar et al.Targeted apoptosis of senescent cells restores tissue homeostasis in response to Chemotoxicity and agingCell(2017)
- D.J. Baker et al.Clearance of p16Ink4a-positive senescent cells delays ageing-associated disordersNature(2011)
- E. Bejarano et al.Defective recruitment of motor proteins to autophagic compartments contributes to autophagic failure in agingAging Cell(2018)
- M.R. Bennett et al.Basic research: killing the old: cell senescence in atherosclerosisNat. Rev. Cardiol.(2016)
- R. Bhat et al.Astrocyte senescence as a component of Alzheimer’s diseasePLoS One(2012)
- A. Bielak-Zmijewska et al.A comparison of replicative senescence and doxorubicin-induced premature senescence of vascular smooth muscle cells isolated from human aortaBiogerontology(2014)
- D.G.A. Burton et al.Obesity and type-2 diabetes as inducers of premature cellular senescence and ageingBiogerontology(2018)
- T.J. Bussian et al.Clearance of senescent glial cells prevents tau-dependent pathology and cognitive declineNature(2018)
- J. CampisiCancer and ageing: rival demons?Nat. Rev. Cancer(2003)
- J. CampisiCellular Senescence and Lung Function during Aging. Yin and YangAnn. Am. Thorac. Soc.(2016)
- J. Campisi et al.Cellular senescence: when bad things happen to good cellsNat. Rev. Mol. Cell Biol.(2007)
- S. Chakradeo et al.Is senescence reversible?Curr. Drug Targets(2015)
- J. Chang et al.Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in miceNat. Med.(2016)
- Z. Chen et al.Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesisNature(2005)
- B.G. Childs et al.Senescent intimal foam cells are deleterious at all stages of atherosclerosisScience(2016)
- B.G. Childs et al.Cellular senescence in aging and age-related disease: from mechanisms to therapyNat. Med.(2015)
- Z.V. Chitikova et al.Sustained activation of DNA damage response in irradiated apoptosis-resistant cells induces reversible senescence associated with mTOR downregulation and expression of stem cell markersCell Cycle, United States(2014)
- M. Collado et al.Tumour biology: senescence in premalignant tumoursNature(2005)
- J.P. Coppe et al.Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressorPLoS Biol.(2008)
- M.C. Cupit-Link et al.Biology of premature ageing in survivors of cancerESMO Open(2017)
- A. Currais et al.Fisetin reduces the impact of aging on behavior and physiology in the rapidly aging SAMP8 mouseJ. Gerontol. A Biol. Sci. Med. Sci.(2018)
- A.R. Davalos et al.Senescent cells as a source of inflammatory factors for tumor progressionCancer Metastasis Rev.(2010)
- M. Demaria et al.Cellular senescence promotes adverse effects of chemotherapy and Cancer relapseCancer Discov.(2017)
- D. Demirovic et al.Curcumin induces stress response and hormetically modulates wound healing ability of human skin fibroblasts undergoing ageing in vitroBiogerontology(2011)
- G.P. Dimri et al.A biomarker that identifies senescent human cells in culture and in aging skin in vivoProc Natl Acad Sci U S A(1995)
There are more references available in the full text version of this article.
Cited by (39)
- Inflammaging: The ground for sarcopenia?2022, Experimental GerontologyShow abstract
- Therapy-induced polyploidization and senescence: Coincidence or interconnection?2022, Seminars in Cancer Biology
- Aging principles and interventional perspectives2022, Plant Bioactives as Natural Panacea against Age-Induced Diseases: Nutraceuticals and Functional Lead Compounds for Drug Development
- A common signature of cellular senescence; does it exist?2021, Ageing Research Reviews
- Senescent cells in cancer therapy: why and how to remove them2021, Cancer LettersCitation Excerpt :Thus, it is of great significance to eliminate these senescent cells. Senotherapy is a promising approach emerged in recent years, which refers to selective removal of senescent cells [98]. The use of senotherapy in combination with currently used cancer therapies has been proposed as a more effective strategy for the treatment of cancer
- Molecular mechanisms and cardiovascular implications of cancer therapy-induced senescence2021, Pharmacology and TherapeuticsCitation Excerpt :Additionally, developing more quantitative tools to estimate senescent cell burden will enable selective treatment of cancer survivors with high senescence burden following cancer treatment. Detection of local senescence, for example, in specific cell types, can provide a better tool to target these particular cells since the efficacy of anti-senescence strategies has been shown to be cell-type dependent (Sikora, Bielak-Zmijewska, & Mosieniak, 2019). Addressing these challenges will ultimately open the door to a new era of interventional research and clinical care that improves the lives of cancer survivors across the lifespan.
View all citing articles on Scopus
Recommended articles (6)
- Research articleClinical strategies and animal models for developing senolytic agentsExperimental Gerontology, Volume 68, 2015, pp. 19-25Show abstract
- Research articleTargeted delivery strategy: A beneficial partner for emerging senotherapyBiomedicine & Pharmacotherapy, Volume 155, 2022, Article 113737Show abstract
- Research articleSenotherapies: A novel strategy for synergistic anti-tumor therapyDrug Discovery Today, Volume 27, Issue 11, 2022, Article 103365Show abstract
- Research articleSenotherapy: A New Horizon for COPD TherapyChest, Volume 158, Issue 2, 2020, pp. 562-570Show abstract
- Research articleTwo-Step Senescence-Focused Cancer TherapiesTrends in Cell Biology, Volume 28, Issue 9, 2018, pp. 723-737Show abstract
- Research articleReducing Senescent Cell Burden in Aging and DiseaseTrends in Molecular Medicine, Volume 26, Issue 7, 2020, pp. 630-638Show abstract




